Royal College of Ophthalmologists Guidelines (Focus) |
Retinopathy of Prematurity |
| Retinopathy
of Prematurity (ROP) is an important cause of childhood blindness that
can be screened for and treated with some success. The sequelae of untreated
or non-responsive ROP can be devastating and may often be associated with
other disabilities, and so steps to limit the condition are of vital importance
for the individual children concerned, for their families and for the healthcare
system that provides for them.
Definition and Classification
Threshold ROP is 5 contiguous clock hours of stage 3 retinopathy in zones I or II (8 clock hours or more if not contiguous), in the presence of 'plus' disease (dilated retinal arterioles and congested veins, abbreviated as '+'). If untreated, it is associated with a poor anatomical outcome in approximately 50% of cases. Pre-threshold ROP is usually defined as any retinopathy in zone 1, or Stages 2+ or 3 in zone II. Stage 3 ROP has a greater likelihood of an anatomically unfavourable outcome if present in the zone 1 (59% poor outcome)than in zone II (44% poor outcome if stage 3+, <1% if no '+' or stage 2). The degree (or lack of) vessel development when first seen and the presence or not of dilated iris vessels, are also both related to the likelihood of a poor outcome, independently of birthweight or gestation. Epidemiology The multicentre Cryotherapy for ROP trial (CRYO-ROP), recruited a prospective cohort of 4099 infants with birthweights less than 1251 grams, at 23 participating centres in the USA (2). Some degree of ROP developed in 66% of the infants overall, but in 85% of these the condition regressed without severe sequelae. The incidence and severity of ROP were related to birthweight and gestation. There was no difference between male and female infants with respect to the occurrence or severitv of ROP. However, white infants were at greater risk than black infants of developing ROP (Odds Ratio 1.30) and threshold ROP (Odds Ratio 2.76), after controlling for birthweight, gestational age, singleton or multiple birth and centre in which born. Recently, mis-sense mutations in the gene responsible for Norries diseases (which is phenotypically similar to stage 4 and 5 ROP) has been identified in 4 of 16 children with advanced ROP for whom molecular genetic analysis was carried out (3). The evidence for racial differences in susceptibility and possible genetic causes in some cases, together with molecular studies on angiogenesis may suggest that '2 hits' are necessary for advanced ROP and further research is ongoing. (3) Screening for ROP Infants should be screened for ROP if they weigh less than 15OOg or were born at 31 weeks or less gestational age. The recommendations from the Royal College of Ophthalmologists and the British Paediatric Association were that screening should begin at 6 - 7 weeks post natally and should continue 2-weekly until retinal vessels are seen in zone III (4). The frequency increases to weeklv if ROP develops until it regresses or gets to threshold level, at which point treatment should be offered. More recently, the American Academy of Paediatrics, the American Association for Paediatric Ophthalmology and Strabismus and the American Academy of Ophthalmology have jointly recommended that screening should begin between 4 6 weeks post natally or 31 - 33 weeks of postconceptional age, whichever is sooner (5). Some ophthalmologists treat pre-threshold (any stage 3 disease) disease in zone I. Screening examinations are usually carried out by ophthalmologists with particular expertise in ROP, using indirect ophthalmoscopy and an indenter to visualise the peripheral retina. The examination is stressful for the infants, whose blood pressures may increase and oxygen saturations decrease, during the instillation of mydriatic drops and retinal examination (6). Stress during the examination may be reduced by using 'nesting' (a form of extra support and padding which still allows free movement) (7). It has recentlv been suggested that non-ophthalmologists might be trained to screen at risk infants for retino-vascular abnormalities, especially in situations or countries where an ophthalmologist might not be available. Such screening was highly sensitive (100%) for abnormal arterioles, but it also involved 16/95 infants being identified as abnormal by the non-ophthalmologist, but then found to be normal by the ophthalmologist. Treatment A recent Cochrane review has combined data from the CRYO- ROP study and an earlier trial to derive estimates of the benefit of treatment. The risk of a poor structural outcome after threshold ROP is reduced from 47.9% to 28.1% by cryotherapy and the risk of poor visual outcome after threshold ROP from 63.0% to 50.6%(8). However; visual fields in sighted eyes are smaller after treatment than in control eyes and the CRYO-ROP study found fewer children with normal vision (better than 20/40) after cryotherapy as well as fewer children with poor vision, as compared with untreated children. Other complications of cryotherapy include cataract, conjunctival proliferation and phthisis. Laser photocoagulation is now seen as equally effective as cryotherapy in preventing progression of the ROP and may be more effective for the treatment of zone I disease (9). Myopia may occur less frequently after laser than after cryotherapy. The results of retinal surgery in cryotherapy or laser treated failures are still disappointing. Sequelae of ROP Serious sequelae of ROP include retinal detachment, retinal folds involving the macula and macula ectopia. In a New Zealand population study, 79% of children who developed ROP and 60% of children who were at risk as infants but didn't develop ROP, had some form of visual defect (high refractive error, Strabismus or amblyopia) (10). If ROP does occur, the risk of subsequent high refractive errors is greater in eyes that develop threshold ROP, or cicatricial changes, than in less severely affected eyes. Premature infants are more likely to have deficits in colour vision (blue-yellow), contrast sensitivity and field defects (especially if there is cerebral damage), than infants born at term. Therefore ex-premature children, especially those in whom ROP developed and either regressed or was treated, should be followed regularly through childhood so that their potential visual problems do not go undetected. Cathy
Williams
REFERENCES 1. Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Brit J Ophthalmol 1984;68:690-697. 2. Multicenter trial of cryotherapy for retinopathy of prematurity Preliminary results. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 1988;106(4):471-9. 3. Shastry B, Pendegrast S, Hartzre M, Liu X, Trese M. Identification of Mis-sense Mutations in the Norrie Disease Gene Associated with Advanced Retinopathy of Prematurity. Arch Ophthalmol 1997;115:651-655. 4. The Royal College of Ophthalmologists British Association of Perinatal Medicine. Retinopathy of Prematurity: Guidelines for Screening and Treatment. London, England: The Royal College of Ophthalmologists British Association of Perinatal Medicine; 1995. 5. American Academy of Pediatrics, the American Association for Pediatric Ophthalmology and Strabismus and the American Academy of Ophthalmology. Screening examination of premature infants for retinopathy of prematurity. Ophthalmology 1997(104):888-889. 6. Laws D, Morton C, Weindling M, Clark D. Systemic effects of screening for retinopathy of prematurity. Br J Ophthalmol 1996(80):425-428. 7. Slevin M, Murphy J, Daly L, O'Keefe M. Retinopathy of Prematurity screening, stress related responses, the role of nesting. Br J Ophthalmol 1997;81:762-764 8.Andersen CC, Phelps DL, Peripheral retinal ablation for threshold retinopathy of prematurity in preterm infants. Cochrane Database Syst Rev 2000;2. 9.Noonan C, Clark D. Trends in the management of stage 3 retinopathy of prematurity. Br J Ophthalmol 1996;80:278-281. 10.Darlow B, Clement R, Horwood L, Mogridge N. Prospective study of New Zealand infants with birth weight of less than1500g and screened for retinopathy of prematurity: visual outcome at age7 -8years. Br J Ophthalmol 1997;81:935-940. 1336-1340. Published by the Royal College of Ophthalmologists, 17 Cornwall Terrace, London, NW1 4QW. Tel: 020 7935 0702 Fax: 020 7935 9838 |
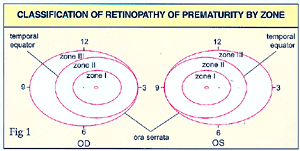 The
study and management of ROP was transformed after the publication of the
International Classification of Retinopathy of Prematurity in 1984 (1).
The severity of the disease is described in stages. Stage I is the development
of a thin, flat, white demarcation line between vascularised and non-vascularised
retina. In stage 2 the line has developed into a ridge, into which vessels
may extend from the retina, but if the extraretinal vessels protrude from
the ridge, stage 3 has been reached. Once extraretinal vessel proliferation
occurs, the fibro-vascular mass may exert traction on the retina and cause
a subtotal retinal detachment which is known as stage 4 (4a if the fovea
is spared and 4b if the fovea is involved). Stage 5 is total retinal detachment,
which is funnel shaped and may be either open or closed at the anterior
and posterior ends. The location of the disease is described in zones.
(See Fig.1).
The
study and management of ROP was transformed after the publication of the
International Classification of Retinopathy of Prematurity in 1984 (1).
The severity of the disease is described in stages. Stage I is the development
of a thin, flat, white demarcation line between vascularised and non-vascularised
retina. In stage 2 the line has developed into a ridge, into which vessels
may extend from the retina, but if the extraretinal vessels protrude from
the ridge, stage 3 has been reached. Once extraretinal vessel proliferation
occurs, the fibro-vascular mass may exert traction on the retina and cause
a subtotal retinal detachment which is known as stage 4 (4a if the fovea
is spared and 4b if the fovea is involved). Stage 5 is total retinal detachment,
which is funnel shaped and may be either open or closed at the anterior
and posterior ends. The location of the disease is described in zones.
(See Fig.1).